PANS: Diagnosis and Treatment
“The diagnosis of PANS should be considered whenever symptoms of OCD, eating restrictions or tics start suddenly, and are accompanied by other emotional and behavioral changes, frequent urination, motor abnormalities and/or handwriting changes.” 3
“Early identification and treatment [of PANS/PANDAS] improve the course of illness and its immediate and likely, long-term impact.” 4
PANS is a clinical diagnosis. Whereas, the Cunningham Panel™ of tests can assist clinicians by providing laboratory evidence of an underlying autoimmune dysfunction and helping determine an appropriate treatment regimen.
One study found, “Antineuronal antibodies (lysoganglioside, tubulin, dopamine receptors, CaM kinase II receptor-stimulating) [as measured with the Cunningham Panel™] that react with human basal ganglia have been found in patients with Sydenham chorea, Tourette syndrome, and PANDAS.” 5
LEARN ABOUT TESTING
PANS: Diagnostic Criteria
- An abrupt, acute, dramatic onset of obsessive-compulsive disorder or severely restricted food intake
“Approximately 1 in 5 children with PANS will have restricted intake of specific foods or all food groups, often with observable weight loss…” 3
“The acuity of symptom onset and age at onset can distinguish PANS-related eating restrictions from more typical anorexia nervosa.” 3
- Concurrent presence of additional neuropsychiatric symptoms with similarly severe and acute onset from at least 2 of the following categories:
- Anxiety
- Emotional Lability and/or Depression
- Irritability, Aggression, and/or Severe Oppositional Behaviors
- Behavioral (Developmental) Regression
- Sudden Deterioration in School Performance
- Motor or Sensory Abnormalities
- Somatic Signs and Symptoms, including Sleep Disturbances, Enuresis, or Urinary Frequency
“The presenting symptoms often change over the first weeks of illness.” 3
- Symptoms are not better explained by a known neurologic or medical disorder
- No age requirement
“PANS has no age limitation, but symptoms typically begin during the grade-school years.” 3
PANS: Treatment Guidelines
Treatment guidelines for PANS and PANDAS were published in 2017 in the Journal of Child and Adolescent Psychopharmacology 6,7,8 The guidelines focused on three treatment areas: psychiatric and behavioral interventions, use of immunomodulatory therapies, and treatment and prevention of infections.
PANS/PANDAS treatment begins with identifying and treating any triggering infections, and addressing the immune dysfunction and inflammation with immune-modulating therapies. 4
“The general ‘principles’ used to treat other brain inflammatory diseases (AE [autoimmune encephalitis], NPSLE [neuropsychiatric lupus], etc.) likely apply to PANS (especially those presenting with severe symptoms):
- Patients given immunotherapy do better and relapse less frequently than patients given no treatment;
- Patients given early treatment do better;
- When patients fail first-line therapy, second-line therapy improves outcomes and reduces relapses.” 6
Furthermore, “Immunomodulatory therapy should be considered early, because NSAIDs [non-steroidal anti-inflammatory drugs] or a short course of oral corticosteroids may be sufficient for symptom remission in recent-onset cases, whereas those with long-standing symptoms often require more intensive and prolonged immunotherapeutic interventions.” 6
- Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol. 2015;25(1):38-47. doi:10.1089/cap.2014.0081 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4340335/
- Kiki Chang, Jennifer Frankovich, Michael Cooperstock, Madeleine W. Cunningham, M. Elizabeth Latimer, Tanya K. Murphy, Mark Pasternack, Margo Thienemann, Kyle Williams, Jolan Walter, Susan E. Swedo, and From the PANS Collaborative Consortium. Journal of Child and Adolescent Psychopharmacology.Feb 2015.3-13.http://doi.org/10.1089/cap.2014.0084 https://www.liebertpub.com/doi/full/10.1089/cap.2014.0084
- PANDAS Physicians Network (PPN). https://www.pandasppn.org/pans/
- Margo Thienemann, MD, Jennifer Frankovich, MD, MS. Sudden Onset of Tics, Tantrums, Hyperactivity, and Emotional Lability: Update on PANS and PANDAS. Psychiatric Times. April 25, 2017.
- Cutforth T, Demille MMC, Agalliu I, Agalliu D. CNS autoimmune disease after Streptococcus pyogenes infections: animal models, cellular mechanisms and genetic factors. Future Neurol. 2016;11:63-76. https://pubmed.ncbi.nlm.nih.gov/27110222/
- Margo Thienemann, Tanya Murphy, James Leckman, Richard Shaw, Kyle Williams, Cynthia Kapphahn, Jennifer Frankovich, Daniel Geller, Gail Bernstein, Kiki Chang, Josephine Elia, and Susan Swedo. Journal of Child and Adolescent Psychopharmacology. Sep 2017.566-573.http://doi.org/10.1089/cap.2016.0145 https://www.liebertpub.com/doi/full/10.1089/cap.2016.0145
- Jennifer Frankovich, Susan Swedo, Tanya Murphy, Russell C. Dale, Dritan Agalliu, Kyle Williams, Michael Daines, Mady Hornig, Harry Chugani, Terence Sanger, Eyal Muscal, Mark Pasternack, Michael Cooperstock, Hayley Gans, Yujuan Zhang, Madeleine Cunningham, Gail Bernstein, Reuven Bromberg, Theresa Willett, Kayla Brown, Bahare Farhadian, Kiki Chang, Daniel Geller, Joseph Hernandez, Janell Sherr, Richard Shaw, Elizabeth Latimer, James Leckman, Margo Thienemann, and PANS/PANDAS Consortium. Journal of Child and Adolescent Psychopharmacology. Sep 2017.574-593.http://doi.org/10.1089/cap.2016.0148 https://www.liebertpub.com/doi/full/10.1089/cap.2016.0148
- Michael S. Cooperstock, Susan E. Swedo, Mark S. Pasternack, Tanya K. Murphy, and for the PANS/PANDAS Consortium. Journal of Child and Adolescent Psychopharmacology. Sep 2017.594-606.http://doi.org/10.1089/cap.2016.0151 https://www.liebertpub.com/doi/full/10.1089/cap.2016.0151
PANS symptoms can be triggered by various infections and is not limited to strep.
Learn More About PANS & Pandas and The Cunningham Panel™
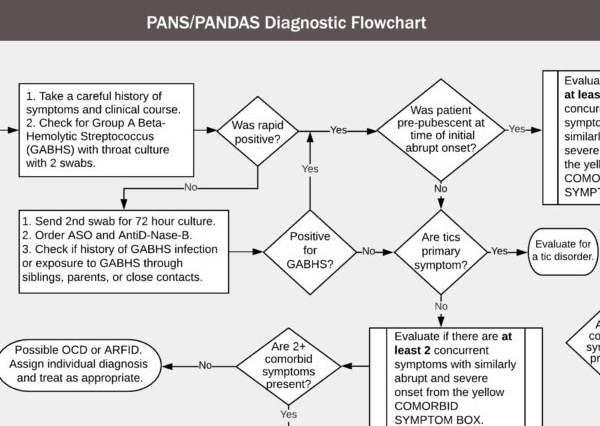
PANS Diagnostic Guidelines

PANS and PANDAS Diagnostic and Treatment Flowchart