
Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism
Published: Translational Psychiatry
Study results suggest that the autoimmune targets in the Cunningham Panel™ can predict IVIG treatment response in a subset of autism patients.
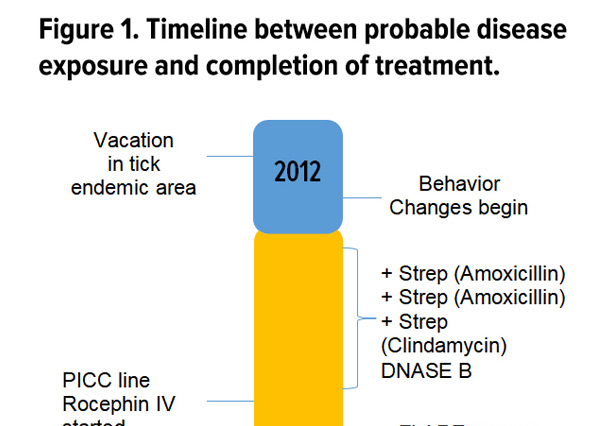
Case Report: PANDAS and Persistent Lyme Disease With Neuropsychiatric Symptoms: Treatment, Resolution, and Recovery
Published: Frontiers in Psychiatry
A previously asymptomatic, healthy 7-year-old girl experienced an abrupt onset of several physical, neurological, and psychiatric symptoms increasing in intensity over a 3-week period.

Autoantibody biomarkers for basal ganglia encephalitis in Sydenham chorea and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections
Published: Frontiers in Psychiatry
Dr. Madeleine Cunningham, co-author, reviews the utilization of antineuronal antibodies (measured with the Cunningham Panel™) as biomarkers for infection-triggered, autoimmune, basal ganglia encephalitis and the significance of the CaMKinase II.

Anti-lysoganglioside and other anti-neuronal autoantibodies in post-treatment Lyme Disease and Erythema Migrans after repeat infection
Published: Brain, Behavior & Immunity
Cunningham Panel™ results indicate Lyme disease may trigger an autoimmune dysfunction, as elevated neuronal autoantibodies have been associated with persistent symptoms.
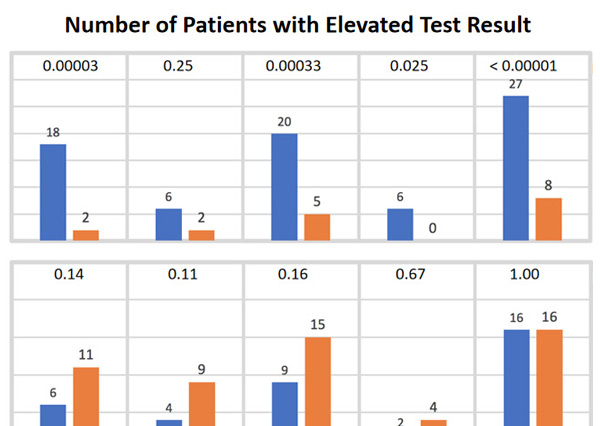
Evaluation of the Cunningham Panel™ in PANDAS and PANS: Changes in antineuronal antibody titers parallel changes in patient symptoms
Published: Journal of Neuroimmunology
Dr. Craig Shimasaki, CEO and President, Moleculera Labs, reviews findings from the study, which describes the sensitivity, specificity and accuracy of the Cunningham Panel™ for patients with PANS and PANDAS.

An atypical presentation of Pediatric Acute Neuropsychiatric Syndrome responding to plasmapheresis treatment
Published: Case Reports in Psychiatry
An atypical presentation of Pediatric Acute Neuropsychiatric Syndrome responding to plasmapheresis treatment. Case review: Dr. Shimasaki





