Cunningham Panel™
Prescribers: Ordering The Cunningham Panel™
Steps to Order The Cunningham Panel™
-
Prescriber Portal
We have a secure Prescriber Portal for clinicians practicing in the U.S. The portal allows practitioners to order tests and retrieve and review results.
Create a profile in the Prescriber Portal.
Place an order for the Cunningham Panel™ and submit the patient’s information.
-
Specimen Collection Supplies
We will send specimen collection supplies by express shipping to the patient address provided, which may require about 2-3 business days to arrive, depending on the patient’s location.
Clinicians who will perform the blood collection at their facility may order specimen collection supplies through the online portal or by calling us at (405) 239-5250.
-
Specimen Collection
The patient will need to have blood collected at your site or an alternate phlebotomy location. IMPORTANT: Blood must be collected into the two glass red-top tubes provided, the serum separated and transferred to the serum transfer tube provided. The Red Top Glass tubes (provided) are the only validated tubes for this panel. Blood collected in any other tubes must be rejected. In cases where the blood cannot be centrifuged, whole blood can be accepted (refer to the Specimen Collection Instructions included with the collection supplies for details).
-
Specimen Return
The serum sample and the completed patient forms (provided in the specimen collection supplies) must be sent to Moleculera Labs overnight (shipping label provided, and shipping costs covered by Moleculera).
IMPORTANT INFORMATION:
- SPECIMENS MUST ARRIVE ON A MOLECULERA BUSINESS DAY. Our facility is closed and unable to receive specimens on weekends and holidays.
- The ice packs provided with the specimen collection supplies must be frozen for a minimum of 24 hours prior to shipping.
- Specimens that arrive warm will be rejected.
Patient results are uploaded to our secure Prescriber Portal and you will be notified by e-mail when results are available. Due to the complexity and number of tests, our turn-around-time may vary. Please click here for our current turn-around-time. Click here to log-in to the Prescriber Portal and order the Cunningham Panel™.
Moleculera can also accept orders written on a prescription form. Prescription form orders must include the following information:
- Provider name, address, phone/fax number, email (for results) and NPI.
- Patient full name, sex assigned at birth and date of birth.
- Responsible party name, email address, and phone number.
- ICD-10 Diagnosis code/codes
Practitioners in New York, please note:
Under New York regulations, practitioners in New York State may order the Cunningham Panel™ for their patients, but specimens must be collected at a facility outside the State of New York. At this time, Moleculera Labs is not licensed in the State of New York and as a result is unable to accept specimens collected in New York.
Pricing and Reimbursement
The total cost of the test is $995. The patient is ultimately responsible for the full cost of the test. For patients with commercial (private or employer group) insurance, a deposit of $495 is required. Moleculera will file a claim to the patient’s insurance carrier as a courtesy. Patients on government-sponsored plans such as Medicaid, TriCare, and Medicare, must pay in full. Moleculera is unable to file claims to any government funded programs. Payment arrangements are available on a case-by-case basis and the patient can contact our office for assistance.
Learn more about how infections can trigger neuropsychiatric symptoms

Cunningham Panel™ helps identify an autoimmune disorder in child initially diagnosed with schizophrenia
Researchers describe a complex case involving a 15-year-old girl, who abruptly developed multiple neurologic and psychiatric symptoms.
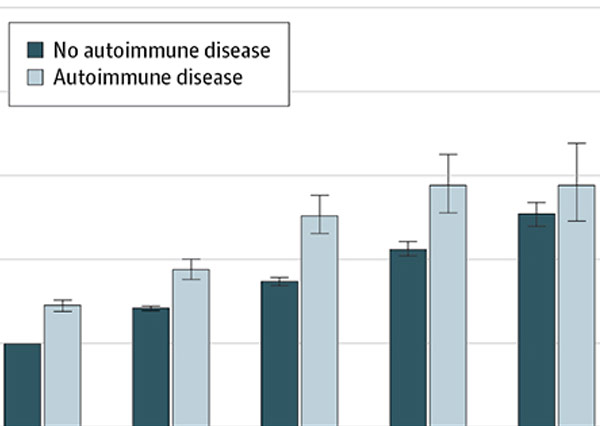
Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study
This nationwide, population-based, prospective cohort study examines the link between mood disorders, infections, and autoimmune disease.
Cunningham Panel™ predicts IVIG treatment response in autistic patients with autoimmune encephalopathy
Case series examines the link between autism spectrum disorders, immune dysfunction, and treatment response to IVIG.