Case Report: PANDAS and Persistent Lyme Disease With Neuropsychiatric Symptoms: Treatment, Resolution, and Recovery
Published: Frontiers in Psychiatry
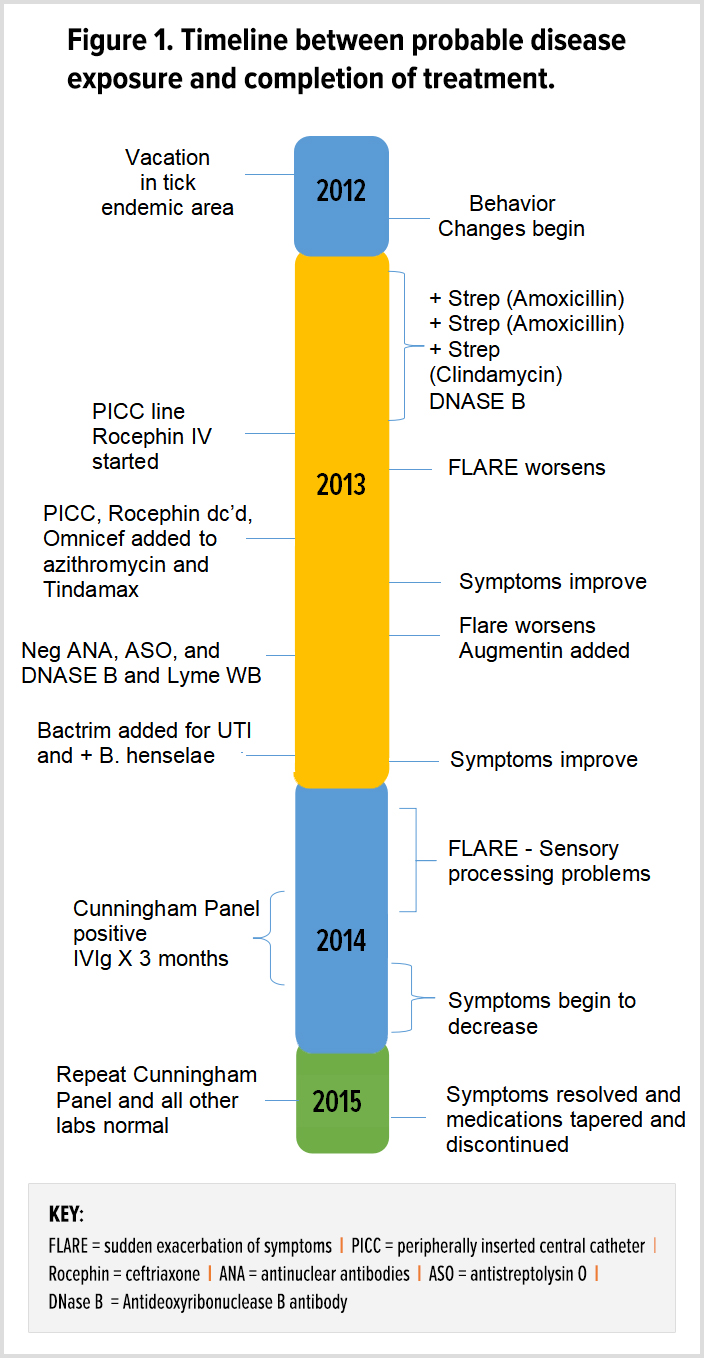
A previously asymptomatic, healthy 7-year-old girl experienced an abrupt onset of several physical, neurological, and psychiatric symptoms increasing in intensity over a 3-week period.
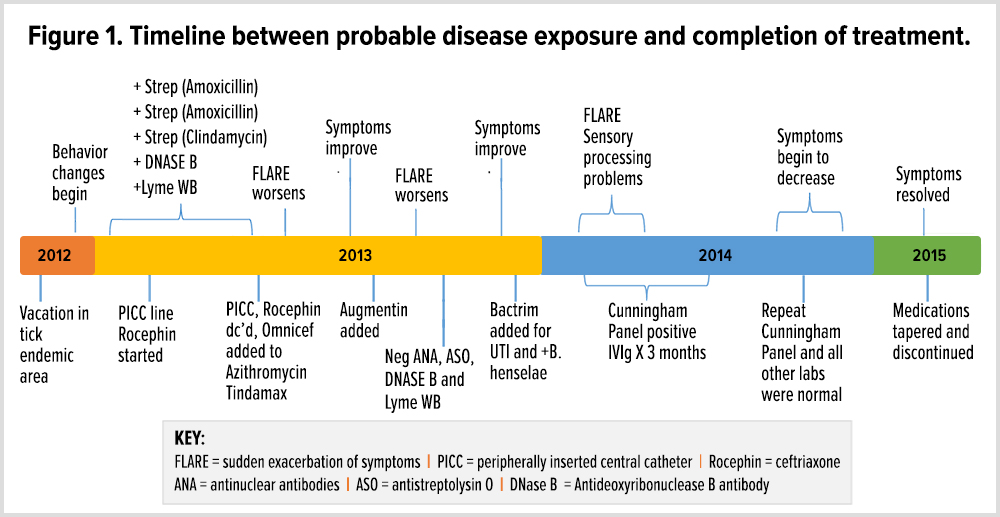
Strep pharyngitis was diagnosed by a previous physician on three separate occasions with the first episode occurring 180 days prior to the onset of neuropsychiatric symptoms.
She was initially treated with amoxicillin with no improvement in symptoms. She was then prescribed a course of clindamycin with a notable improvement in the patient's symptoms.
The patient was referred to a psychiatric department. However, the mother chose to have the child evaluated by a developmental pediatrician who diagnosed her with PANDAS, pediatric autoimmune neuropsychiatric disorders associated with strep infections.
Further testing revealed the young girl was positive for Lyme disease (CDC Western blot).
On her first visit with the pediatric Lyme disease specialist, the patient presented with crying, anxiety, headache, joint pain, decreased cognitive functioning, fatigue, nighttime awakening and an extreme fear of sleeping alone.
Over the course of her illness, the patient was treated with a range of medications including IV Rocephin, Omnicef, Zithromax, Tindamax, Augmentin, and Bactrim.
At her 8-week follow-up visit, the mother reported an overall improvement in her child’s symptoms with no headaches, but increased insomnia and scattered joint sensitivities.
However, over the following months, the patient experienced a waxing and waning of various symptoms including facial “twitching,” depression, hyperactivity, and oppositional behavior.
Test results were positive for Babesia duncani and the patient was treated with Mepron.
At her 4-month follow-up appointment, the patient’s mother reported that her child was having panic attacks and was “terrified” to sleep alone. She had some residual combativeness, lack of focus, and cognitive interference.
The Cunningham Panel™ was ordered to assess the presence of antineuronal antibodies against specific neuronal receptors.
The patient's Anti-Dopamine D1 Receptor (DRD1), Anti-Dopamine D2L Receptor (DRD2L), and Anti-Tubulin (TUB) were elevated. Her Anti-Lysoganglioside-GM1 (LYSO-GM1) and Calcium/calmodulin-dependent protein kinase II (CaMKII) were normal.
Published studies demonstrated that the elevated presence of one or more of these antineuronal antibodies and antibody mediated stimulation of CaMKII was strongly associated with autoimmune neuropsychiatric symptoms such as those present in PANDAS and PANS.
Based upon Cunningham Panel test results, the patient began intravenous immunoglobulin (IVIG) treatment.
A follow-up Cunningham Panel test was performed to assess the post-treatment status of antineuronal antibodies. All 5 assay were within normal range and the clinician determined that no further IVIG treatment was needed.
This patient appears to be fully recovered. She is asymptomatic and performing academically at the “top” of her class according to her mother.
The author’s concluded, “As evidenced by her recovery and resolution of symptoms, treating both the Lyme infection and streptococcal infection, as well as treating the underlying autoimmune etiology of her neuropsychiatric symptoms resulted in a successful outcome.”
Furthermore, “The presence of elevated antineuronal antibodies identified by the Cunningham Panel™ provided an aid in the diagnosis and in directing immunomodulatory treatment.”
“The post-treatment resolution of these autoantibodies provided pathophysiological support for addressing both the infection(s) and the underlying immune system dysfunction which resulted in a positive medical outcome for this patient.”
Learn More About PANS & Pandas and The Cunningham Panel™

PANS: Diagnosis and Treatment

Pandas & PANS Patient Stories